Institute for Safe Medication Practices, Horsham, PA
A prescriber ordered vaccinia immune globulin intravenous, human (Vigiv–Cangene Corporation) 6,000 units/kg for a hospitalized 3.49 kg neonate (total dose of 20,940 units) with mpox-like symptoms. A single-dose vial arrived from the national stockpile in an unlabeled carton without a package insert.
The vial label displayed, “greater than or equal to 50,000 units per vial,” without listing a corresponding volume or concentration. When trying to determine how to prepare this product, a pharmacist found the package insert on DailyMed on the NIH website, which states that Vigiv is provided in a 20-mL single-dose vial containing antibodies to vaccinia virus at greater than or equal to 50,000 units per vial. The package insert states to remove the entire contents of the vial to obtain the labeled dosage of Vigiv. Practitioners might assume the vial contains a total volume of 20 mL equaling 50,000 units, but 20 mL is the size of the glass vial. The actual volume in each vial is variable.
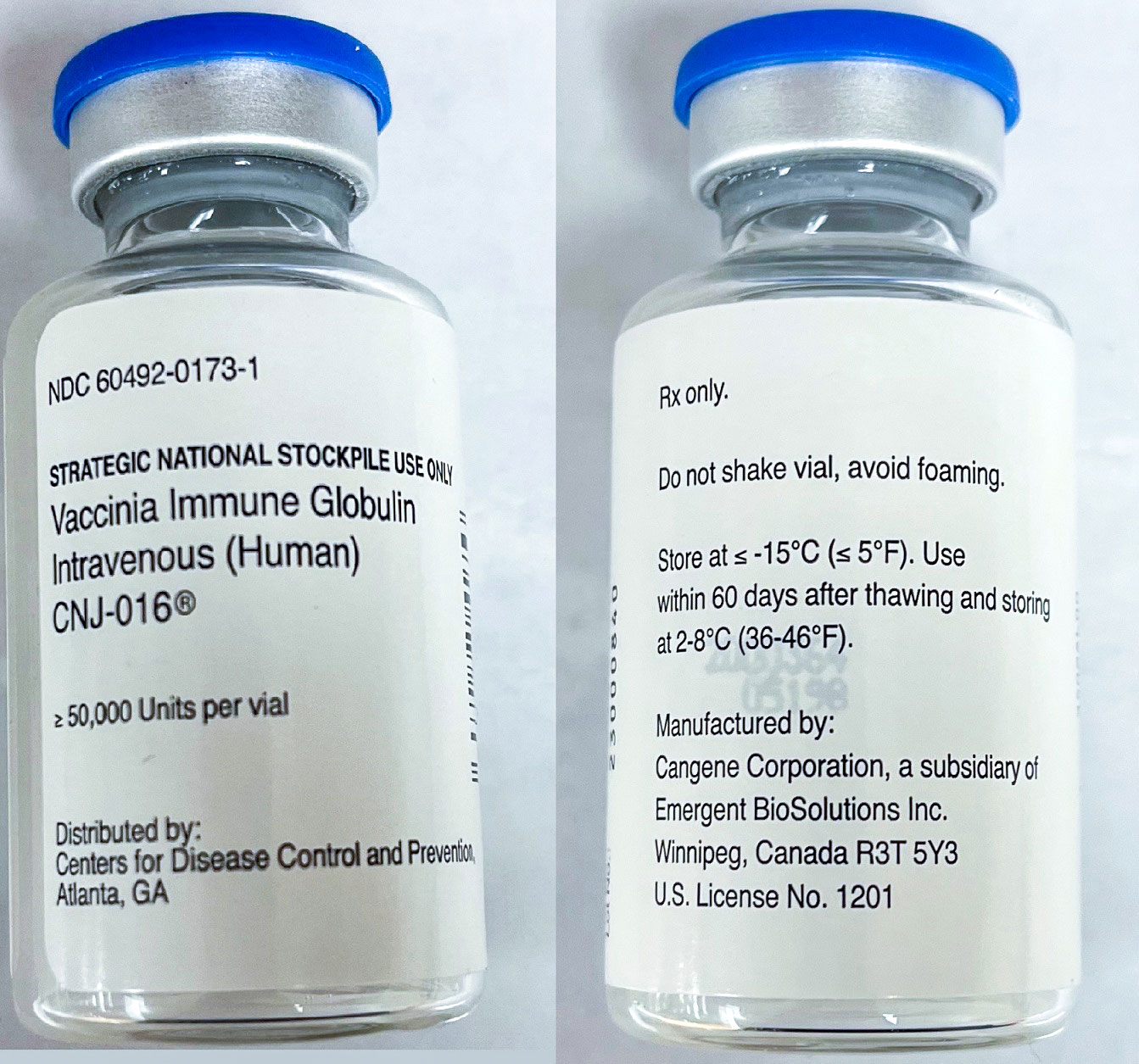 The vaccinia immune globulin intravenous (human) CNJ-016 vial label displays greater than or equal to 50,000 units per vial, without a corresponding volume or concentration.
The vaccinia immune globulin intravenous (human) CNJ-016 vial label displays greater than or equal to 50,000 units per vial, without a corresponding volume or concentration.
The pharmacist consulted with CDC and was instructed to withdraw the total volume in the vial, then divide 50,000 units by the volume to determine the final concentration. The total volume was determined to be 11.5 mL and resulted in a concentration of 4,348 units/mL. Therefore, the patient-specific dose (20,940 units) was calculated to be 4.82 mL. If the CDC specialist had not been available, the pharmacist might have incorrectly determined the concentration to be 50,000 units/20 mL (2,500 units/mL), which would have resulted in a final dose of 36,436 units/8.38 mL, almost double the intended dose.
Before the pharmacist could verify the order in the electronic health record and generate a patient-specific label, which requires the volume and concentration, the technician had to first draw up the volume of the vial to determine the concentration. This is another opportunity for error because the technician will not have a printed label for the syringe.
ISMP has confirmed this unusual situation with CDC. The vials are filled from pooled plasma with a minimum of 50,000 units per vial, thus the volume and concentration per vial vary. When this medication is requested and approved to treat mpox, CDC emails an investigational new drug (IND) protocol to the prescriber in advance, and this should be distributed to pharmacy staff for reference in dose preparation and titration instructions.
In addition to the container label issue, the “administration” section of the package insert states that Vigiv should be given intravenously at an infusion rate no greater than 2 mL/minute. For patients weighing less than 50 kg, it should be administered at a rate no greater than 0.04 mL/kg/minute (133.3 units per kg/minute). However, when the pharmacist consulted with CDC, they learned about the IND protocol which indicated that for certain patients, Vigiv administration should be initiated at an infusion rate of 0.01–0.02 mL/kg/minute for the first 30 minutes and then it can be increased by 0.01–0.02 mL/kg/minute from the initial infusion rate for the next 30 minutes. After that time, the remaining infusion may be administered at a rate of 2 mL/minute.
If a patient requires the use of Vigiv, obtain the current IND protocol from the prescriber or CDC, consider creating a worksheet to calculate the concentration in the vial in hand, and include a label to use when drawing up the vial contents. Include complete titration instructions for the nurse so that Vigiv is administered at the correct rate. ■